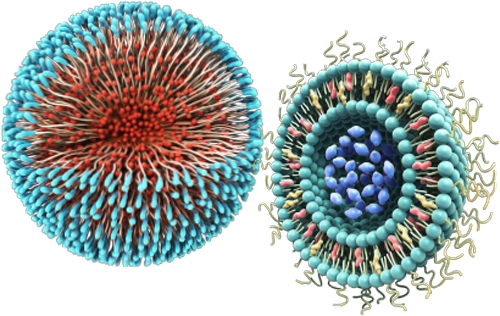
In spite of the opportunities they bring, nanomedicines are highly complex and their quality attributes are closely linked to their manufacturing process. Changes in quality, safety, efficacy, size distribution, surface properties, drug loading and release profile, aggregation status and stability – can alter how a nanomedicine acts within the body with a significant impact on patient safety and efficacy.
In order to ensure their safety and efficacy, it is therefore essential that a robust regulatory process exists and that all stakeholders, including health authorities, payers, pharmacists and prescribers are fully aware of their complexities.
3 key recommendations to ensure patient safety and enable the EU to efficiently fully harness the potential of nanomedicines: